Abstract
Monoalleleic inactivating mutations in histone acetyltransferase (HAT) enzymes promote lymphomagenesis in germinal center derived B-cell lymphomas, follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL), occurring in about 40% of patients. The intact wild-type allele offers an opportunity to leverage the normal enzyme to overcome the pathogenic impact of the mutated allele. We hypothesize that if inactivating mutations in HATs are critical to FL and DLBCL lymphomagenesis, then drugs capable of inducing enhanced function of the wild-type HAT allele product should be cytotoxic in cells harboring HAT mutations.
We designed and synthesized a library of new chemical entities with HAT activating properties (N=70). The cytotoxic effects of the compounds (N=29) were evaluated via medium-throughput screening in 4 DLBCL cell lines. IC50 ranged from 3.6 to 43.2 µM. Focusing on 6 analogue compounds, which share the same Nphenylbenzamide scaffold, we evaluated cytotoxicity across an expanded panel of 11 DLBCL cell lines. The median IC50 of 6 analogues tested was lower in the EP300 mutated cell lines (median 9.6 µM, range 5 - 11 µM) compared to the wildtype lines (median, 17 µM, range 15 - 24 µM). YF2 was chosen as the lead compound because it was the most selective of the analogues in inducing cytotoxicity in cell lines harboring EP300 mutations compared to wildtype (IC50 5 µM and 19 µM respectively, p<0.0005).
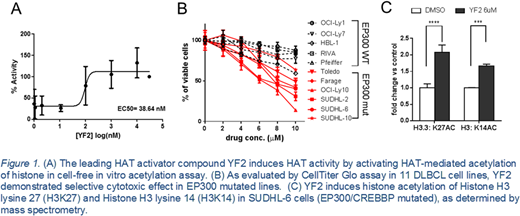
To determine YF2's functional effect on activating p300 in a cell free assay, p300-mediated histone and p53 acetylation was measured by combining recombinant p300, substrate and acetyl-CoA. YF2 increased p300-mediated histone H3 lysine27 acetylation (EC50=38.64 nM) and H3 lysine18 acetylation (EC50=1.656 nM). YF2 also induced acetylation of p53 by 5-fold in a dose dependent manner. In cellular assays, YF2 induces acetylation of histone (H3K27 2-fold and H3K14 1.6-fold) after exposure in the SUDHL-6 cell line (EP300 mutated) as measured by mass spectrometry and confirmed by immunoblot of histone extracts.
To assess the pharmacokinetics (PK) and preliminary in vivo efficacy of YF2, SUDHL-6 (EP300-mutated) xenograft bearing mice were treated once daily i.p. for 6 days with YF2 doses of 40mg/kg or 60mg/kg. Serum and tumor samples were collected at sequential time points. The Cmax of YF2 60mg/kg was 2424 ng/mL (5.1 µM) whereas Cmax of 40mg/kg was 2091.96 ng/mL (4.5 µM) which is consistent with the cellular IC50. Both concentrations of YF2, 40mg/kg and 60mg/kg, accumulated in tumor with Cmax 9536.71 and 9858.15 ng/g, respectively. YF2 is rapidly absorbed in the serum (Tmax 0.25 h) and sustained in the tumor (Tmax 4h). Significant effects on tumor size were observed in 13 of 19 mice demonstrating decreased tumor volume following only 6 days of YF2 treatment. Mice treated with YF2 40mg/kg induced H3K27 acetylation in tumor specimens as determined by mass spectrometry.YF2 40mg/kg was well tolerated in SCID/beige mice for 30 days without significant weight loss, while 60mg/kg YF2 led to 20% weight loss during 6-days of treatment. Additionally, YF2 demonstrated cytotoxic effects in 2 primary patient lymphoma samples but were non-cytotoxic to peripheral blood mononuclear cells from healthy donors.
Furthermore, we hypothesized that if DLBCL is sensitive to an enhanced acetylation state, then combined targeting of epigenetic machinery with HAT activators and HDAC inhibitors may induce profound epigenetic modification leading to synergistic induction of programmed cell death. The concentration : effect relationship of YF2 and the pan-HDAC inhibitor, romidepsin, was evaluated over time across a panel of lymphoma cell lines (N=7). Synergy was calculated by Excess over Bliss (EOB>10 connotes synergy). Combination of YF2 and romidepsin demonstrated strong synergism in DLBCL lines (EOB = 48). The combination led to enhanced histone acetylation compared to either single agents. The combination is safe in mice and murine xenograft studies of the combination are underway.
In summary, YF2 induces HAT-mediated acetylation of histone and p53. It demonstrates selective cytotoxic effects in EP300-mutated DLBCL cell lines, and is both well tolerated and effective in xenograft mouse models of lymphoma suggesting potential clinical application and precision medicine opportunities for patients harboring this mutation.
O'Connor:Seattle Genetics: Research Funding; ADC Therapeutics: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal